ExaWizards and Kyoto University Develop Drug Safety Evaluation AI Learning from PMDA Evaluation Results 〜Promote more efficient reporting and evaluation of pharmaceutical products and reduce the number of cases requiring human evaluation〜
ExaWizards Inc. (Headquarters: Minato-ku, Tokyo; Representative Director & President: Makoto Haruta; hereafter, “ExaWizards”) in collaboration with Kyoto University (hereinafter referred to as “Kyoto University”), has developed an evaluation AI that learns from the results of past evaluations by the Pharmaceuticals and Medical Devices Agency (PMDA) of cases from the “Collection and Analysis of Pharmacy Hiyari-Hatto Cases” (hereinafter referred to as “pharmacy near-miss cases”) published by the Japan Agency for Quality Health Care Evaluation (hereinafter referred to as “the Evaluation Agency”). The evaluation AI has been developed by learning the results of past evaluations by the Pharmaceuticals and Medical Devices Agency of “pharmacy near-miss cases. This evaluation AI is expected to eliminate the number of pharmacy near-miss cases that require countermeasures for pharmaceutical products, and reduce the number of cases requiring human evaluation by 30-60%.

ExaWizards and Kyoto University have developed an AI to evaluate textual report data on drug safety since FY2020 based on the results of PMDA’s evaluation of “pharmacy near-miss cases” such as drug mix-ups that may lead to patient health problems (*1). After additional validation and updating of the evaluation model in FY2022, we have now developed an evaluation AI system that can be expected to be used by non-engineering field investigators. The introduction of an evaluation AI for drug safety that learns from past PMDA evaluation results is expected to facilitate more efficient reporting evaluations and reduce the number of cases subject to human evaluation.
☑︎ Achieving both efficiency and safety through zero oversights and reduced human evaluation
Approximately 100,000 to 180,000 cases of pharmacy near-misses are reported annually, of which 6,000 to 7,000 cases are subject to investigation by PMDA.
PMDA examines the necessity of safety management measures (“product” measures) taken by manufacturers and distributors to address the causes of these cases, and takes necessary measures based on the evaluation results.
The number of cases investigated by PMDA is on the increase, and the efficiency of reporting and evaluation is expected to improve. However, the reporting data includes text data, and it is difficult to automate the evaluation by simply dividing the reporting items into cases. For this reason, the use of AI was expected to improve efficiency.
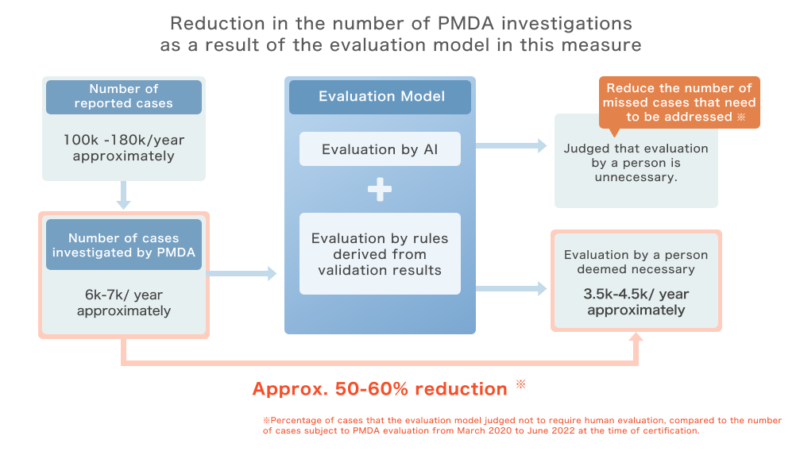
ExaWizards and Kyoto University have jointly developed an AI that evaluates the necessity of safety measures by learning the results of past evaluations conducted by PMDA, and have succeeded in achieving 96% accuracy in Recall, an index that “minimizes oversight” of cases requiring measures as of FY2020 (*1).
In addition, in the validation of the surveyed cases in FY2021 and FY2022, we confirmed that all cases that were deemed to require human evaluation (see figure) were extracted. Subsequently, by developing an evaluation model that combines “evaluation by AI” and “evaluation by rules derived from the verification results,” we have realized that we can reduce the number of cases subject to investigation by PMDA by approximately 30-60% compared to the case where only existing extraction conditions are applied, while ensuring “zero oversight” of cases requiring countermeasures during the verification process (*2).
Furthermore, we have developed the developed evaluation model as a system that can be utilized by non-engineers in the PMDA field. In developing this system, we have utilized the exaBase (*3) platform.
The system will be introduced in practice on a trial basis with human evaluation to confirm its usefulness, and the suitability for full-scale introduction will be considered.
ExaWizards has developed an evaluation AI that eliminates the need to take countermeasures for pharmaceutical “objects” in pharmacy near-miss cases and reduces the number of cases requiring human evaluation. Exerwizards will continue to provide AI solutions that can solve various issues related to medical care and pharmaceuticals, including AI that evaluates drugs by referring to their attribute information and AI that evaluates text data containing drug information.
(*1) August 11, 2021 Press release, “ExaWizards and Kyoto University Jointly Develop AI to Evaluate Text Reporting Data on Drug Safety.
(*2) The ratio of cases for which the evaluation model determined that human evaluation is unnecessary to the number of cases subject to PMDA evaluation from March 2020 to June 2022: ratio combined. PMDA evaluates reported cases every few months, and the reduction ratio fluctuates according to the distribution of case types at each evaluation timing.
(*3) exaBase is an AI platform that provides AI algorithms, APIs, and ready-to-use AI/DX SaaS. Assets are accumulated and improved based on support for over 250 AI and DX projects per year.
*exaBase is a registered trademark of ExaWizards.
☑︎About Exawizards
Since its establishment in 2016, ExaWizards has been consistently developing and implementing AI-enabled services and developing strategies to address social issues. Our business segments are divided into AI platform business and AI product business, and we accumulate proprietary algorithms and data while turning both AI platform and AI product businesses as a multi-sectoral and multi-modal strategy. ExaWizards has applied for 196 patents and has been granted 95 patents (as of March 31, 2023), and also possesses advanced technologies in the hardware domain, such as robots and AI cameras. For more information about ExaWizards’ Care & Med Tech business, please visit https://exawizards.com/care-med-tech.
【Company profile of ExaWizards, Inc.】
Company name: ExaWizards Corporation
Location: 21F, Shiodome Sumitomo Building, 1-9-2 Higashi-Shinbashi, Minato-ku, Tokyo
Representative: Makoto Haruta, President and Representative Director
Business: Industrial innovation and resolution of social issues through the development of services utilizing AI
URL: https://exawizards.com/
<Contact for public relations>
E-mail address of the Public Relations Division of ExaWizards Inc.: publicrelations@exwzd.com